Bcs Classification Of Drugs List

The “” is an FDA guidance document, which allows pharmaceutical companies to forego clinical bioequivalence studies, if their drug product meets the specification detailed in the guidance. The principles of the BCS classification system can be applied to NDA and ANDA approvals as well as to scale-up and post approval changes in drug manufacturing.
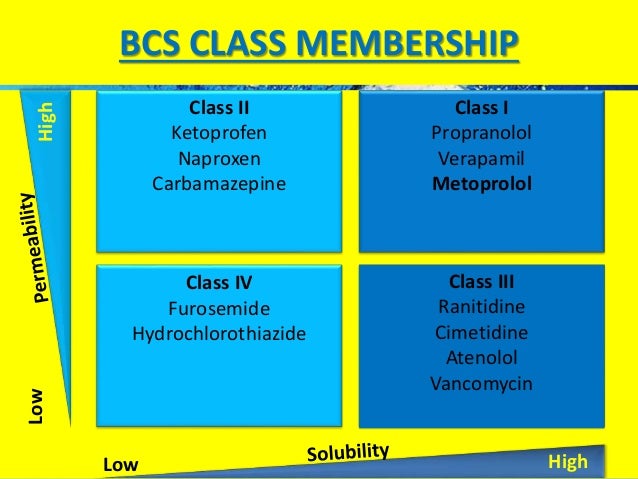
The Biopharmaceutics Classification system (BCS) classifies drug substances based on aqueous solubility and intestinal permeability. The objective of this study was to use the World Health Organization Model List of Essential Medicines to determine the distribution of BCS Class 1, 2, 3, and 4 drugs. GUIDANCE ON BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS)-BASED BIOWAIVER National Pharmaceutical Control Bureau, Ministry Of Health Malaysia. January 2013 Adopted and adapted mainly from the following: 1. Guideline On The Investigation Of Bioequivalence (European Medicines Agency, London, 20 January 2010, CPMP/EWP/QWP/1401/98 Rev.
Bcs Classification Of Drugs List

Fda Bcs Classification List Of Drugs
A waiver of In-vivo Bioavailability and Biioequivalence studies based on the BCS classification can therefore save pharmaceutical companies a significant amount of development time and reduce development costs. The BCS classification system is based on the scientific rationale that, if the highest dose of a drug candidate is readily soluble in the average fluid volume present in the stomach (250 ml) and the drug is more than >85% absorbed, then the in vitro drug product dissolution profiles should allow assessment of the equivalence of different drug formulations. Solubility and dissolution can be easily measured in vitro.